At What Point Does Water In The Air Change From A Gas (Vapor) To A Liquid?

In physics, a vapor (American English) or vapour (English English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,[1] which means that the vapor can exist condensed to a liquid by increasing the pressure on information technology without reducing the temperature. A vapor is different from an droplets.[2] An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas.[2]
For example, h2o has a critical temperature of 647 K (374 °C; 705 °F), which is the highest temperature at which liquid h2o can exist. In the atmosphere at ordinary temperatures, therefore, gaseous water (known as h2o vapor) volition condense into a liquid if its partial pressure is increased sufficiently.
A vapor may co-exist with a liquid (or a solid). When this is true, the 2 phases will exist in equilibrium, and the gas-partial pressure will be equal to the equilibrium vapor pressure level of the liquid (or solid).[1]
Properties [edit]
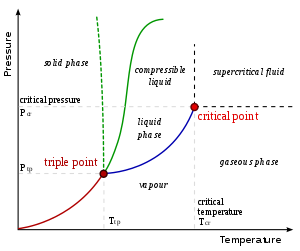
The vapor-liquid critical bespeak in a pressure-temperature phase diagram is at the high-temperature extreme of the liquid–gas phase purlieus. (The dotted green line gives the anomalous behaviour of water.)
Vapor refers to a gas phase at a temperature where the same substance tin can also exist in the liquid or solid country, beneath the critical temperature of the substance. (For example, h2o has a disquisitional temperature of 374 °C (647 Grand), which is the highest temperature at which liquid h2o can exist.) If the vapor is in contact with a liquid or solid phase, the ii phases volition be in a land of equilibrium. The term gas refers to a compressible fluid stage. Fixed gases are gases for which no liquid or solid can form at the temperature of the gas, such as air at typical ambience temperatures. A liquid or solid does not accept to eddy to release a vapor.
Vapor is responsible for the familiar processes of cloud germination and condensation. It is commonly employed to carry out the concrete processes of distillation and headspace extraction from a liquid sample prior to gas chromatography.
The constituent molecules of a vapor possess vibrational, rotational, and translational motion. These motions are considered in the kinetic theory of gases.
Vapor pressure level [edit]


The vapor pressure is the equilibrium force per unit area from a liquid or a solid at a specific temperature. The equilibrium vapor pressure of a liquid or solid is not affected by the amount of contact with the liquid or solid interface.
The normal humid point of a liquid is the temperature at which the vapor pressure is equal to normal atmospheric pressure.[i]
For 2-phase systems (east.g., two liquid phases), the vapor pressure of the individual phases are equal. In the absenteeism of stronger inter-species attractions between like-like or like-unlike molecules, the vapor pressure follows Raoult'southward constabulary, which states that the partial pressure level of each component is the product of the vapor pressure of the pure component and its mole fraction in the mixture. The total vapor pressure is the sum of the component partial pressures.[iii]
Examples [edit]

Invisible water vapor condenses to class visible water droplets chosen mist
- Perfumes contain chemicals that vaporize at dissimilar temperatures and at dissimilar rate in scent accords, known equally notes.
- Atmospheric water vapor is found near the earth's surface, and may condense into small liquid aerosol and form meteorological phenomena, such every bit fog, mist, and haar.
- Mercury-vapor lamps and sodium vapor lamps produce light from atoms in excited states.
- Flammable liquids practise non burn when ignited.[4] It is the vapor cloud above the liquid that will fire if the vapor's concentration is between the lower flammable limit (LFL) and upper flammable limit (UFL), of the flammable liquid.
Eastward-cigarettes produce aerosols, non vapors.[2]
Measuring vapor [edit]
Since it is in the gas phase, the amount of vapor present is quantified past the fractional pressure of the gas. Too, vapors obey the barometric formula in a gravitational field, only every bit conventional atmospheric gases do.
Run across also [edit]
| | Look upwardly vapor or vapour in Wiktionary, the gratis lexicon. |
- Dilution (equation)
- Evaporation – Type of vaporization of a liquid that occurs from its surface; surface phenomenon
- Henry'south police force – Gas police regarding proportionality of dissolved gas
- Contrail, also known equally Vapor trail – Long, thin artificial clouds that sometimes form behind shipping
- Vaporizer (disambiguation)
References [edit]
- ^ a b c R. H. Petrucci, Due west. S. Harwood, and F. G. Herring, General Chemical science, Prentice-Hall, 8th ed. 2002, p. 483–86.
- ^ a b c Cheng, T. (2014). "Chemical evaluation of electronic cigarettes". Tobacco Command. 23 (Supplement 2): ii11–ii17. doi:10.1136/tobaccocontrol-2013-051482. ISSN 0964-4563. PMC3995255. PMID 24732157.
- ^ Thomas Engel and Philip Reid, Concrete Chemistry, Pearson Benjamin-Cummings, 2006, p.194
- ^ Ferguson, Lon H.; Janicak, Dr Christopher A. (2005-09-01). Fundamentals of Burn down Protection for the Safety Professional person. Government Institutes. ISBN9781591919605.
Source: https://en.wikipedia.org/wiki/Vapor
Posted by: davisandessaint.blogspot.com

0 Response to "At What Point Does Water In The Air Change From A Gas (Vapor) To A Liquid?"
Post a Comment